Jeffrey A. Singer
Naloxone has saved tens of thousands of lives. Yet, more than 50 years after the FDA approved it in injectable form, government rules still make the most affordable version hard to access.
The Food and Drug Administration originally approved naloxone as a prescription-only drug in its injectable form in 1971. Health care practitioners and first responders have been using it for decades to reverse opioid overdoses. The FDA approved the prescription-only nasal spray formulation of naloxone in 2015. Several years after many policy experts and I called for the government to allow people to access the drug without a prescription, the FDA finally removed its barriers to over-the-counter naloxone nasal spray in 2023.
Wider access to the overdose antidote naloxone has undoubtedly contributed to the recent decline in opioid-related overdose deaths. Now, many lawmakers, including Republican lawmakers, are unhappy about the White House’s plans to terminate many federal programs that the Substance Abuse and Mental Health Services Administration (SAMHSA) oversees, which distribute and educate people on how to use the naloxone nasal spray.
While I have long praised naloxone’s harm reduction benefits and urged the government to eliminate barriers to its accessibility, there are numerous ways to achieve this without relying on taxpayer funding.
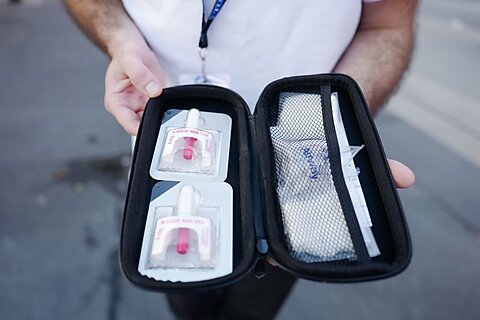
For example, the injectable formulation of naloxone, which individuals can administer intravenously (IV), intramuscularly (IM), or subcutaneously (under the skin; SQ), has been available in generic form for decades and can cost hundreds of dollars less than the nasal spray version of the drug. The nasal spray version is significantly more expensive due to its delivery mechanism’s complexity and long-standing brand dominance. Unfortunately, the FDA still requires individuals to obtain a permission slip from a state-licensed health care practitioner to access it.
All states have developed workarounds to address the FDA’s injectable naloxone prescription barrier. Most states allow prescribers to issue a “standing order,” listing themselves as the prescriber of record to any individual who presents to a pharmacy or harm reduction organization seeking injectable naloxone. This saves individuals the inconvenience and cost of going to a clinic or clinician’s office for a prescription.
In my state of Arizona, I frequently volunteer to issue the standing order to Sonoran Prevention Works, a large and well-established harm reduction organization, which allows them to distribute injectable naloxone to their clients. The organization’s executive director informed me that, although they can buy naloxone nasal spray over the counter, the injectable form is much cheaper and more cost-effective for them to acquire on their tight budget that relies solely on grants and individual donations.
Injectable naloxone has a long track record of safety and is easy to administer with minimal training, often via a simple intramuscular injection. After more than 50 years of experience with injectable naloxone, it is time for the FDA to eliminate its barriers to over-the-counter injectable naloxone. This would expand access to more affordable forms of the antidote.
The FDA can take additional steps by easing its restrictions on compounding pharmacies. The FDA does not regulate compounding pharmacies; state pharmacy licensing boards oversee them. However, the FDA requires compounding pharmacies to adhere to United States Pharmacopeia (USP) standards and to use FDA-approved ingredients from FDA-approved facilities. Unfortunately, the FDA does not allow compounding pharmacies to produce products that are essentially identical to commercially available products unless the agency declares a shortage of that product. The agency only permits pharmacists to compound “patient-specific” formulations of these products.
As I previously discussed regarding GLP-1s, the FDA should permit compounding pharmacies to create products identical to those commercially available, provided patents no longer protect them. This would enhance the availability, affordability, and accessibility of naloxone. Harm reduction organizations could then negotiate with compounding pharmacies to supply them with ample, affordable supplies of naloxone.
If lawmakers are serious about expanding access to naloxone and other harm reduction strategies, they should shift their focus from taxpayer subsidies to removing the many obstacles the government erects that prevent harm reduction organizations and people who use drugs from obtaining them.